loaction
SNU Professor Kisuk Kang's Team Develops a Post-Lithium Secondary Battery That is Published in
-
작성자
관리자
-
등록일
2020.12.31
-
조회수
376
SNU Professor Kisuk Kang's Team Develops a Post-Lithium Secondary Battery That is Published in
<Nature Nanotechnology>
<Nature Nanotechnology>
- With the development of micro-pore control separators, the implementation of organic batteries has been accelerated
- The results show to be utilized in many fields such as in electric vehicles that require high energy density.
- The results show to be utilized in many fields such as in electric vehicles that require high energy density.
(From left) Professor Kisuk Kang of the SNU Department of Materials Science and Engineering,Hybrid Materials, co-first authors Songyan Bai and Byunghoon Kim
A research team led by Professor Kisuk Kang of the SNU Department of Materials Science and Engineering,Hybrid Materials has caught the attention of the world of academia by their development of post-lithium secondary batteries. The research paper was published as of November 2 in the world-renowned journal of <Nature Nanotechnology>.
As demand for sustainable renewable energy has soared, developing an energy storage system (ESS) that stores renewable energy has emerged to become a topic of global interest. Lithium secondary batteries can be used repeatedly for energy charging and discharging processes that use stored energy, and are widely used as ESSs due to their high energy density.
The research has developed a way to overcome the problem of low life expectancy in organic secondary batteries'*, which is being recognized as the next-generation battery material, and its results have greatly increased the possibility of commercialization in the future. Beginning with electric cars that require high energy density, it can also be used as energy storage systems (ESS) and in the long run, could replace current transition metal* based anode materials.
* Organic secondary battery: A secondary battery in which the anode material is composed of organic molecules capable of redox.
* Transition metal: Metal elements corresponding to periods 4-7, groups 3-12 in the periodic table. They are capable of easily carrying out redox.
Organic secondary batteries are in the spotlight as an alternative to transition metals because they are resources that are easy to acquire rand cheap. However, the issue of low life-expectancy had been a stumbling block. The research team revealed that the fundamental reason for the lowered life expectancy is due to the shuttle phenomenon, which is when 'dissolved organic electrode molecules move to the opposite cathode'.
In order to suppress this shuttle phenomenon, a micro-pore-controlled separating membrane was developed that could block the movement of organic electrode molecules as lithium ion and electrolyte molecules passed through the cell. By introducing this structure between the positive and negative poles, they have succeeded in drastically improving the life expectancy of organic secondary batteries.
The research team found that a material called Metal Organic Frameworks (MOFs) consists of a number of uniformly and regularly spread holes within the grid structure, and that the proper selection and combination of metal and organic units, which are the components of MOFs, allows the size of the holes to be adjusted at a sub-nanometer level.
Through the separation membrane's nano-processing, the electrolyte salt molecules create a zeolite imidazole framework-8 (ZIF-8) that can inhibit the movement of organic electrode molecules while allowing the salt molecules to pass through. Through the introduction of the MOFs separation membrane, it was confirmed that the electrode capacity remains at a minimum of 80% even after 2,000 of long-term charge discharge cycles. This demonstrates that when compared to the conventional organic secondary batteries that had been reported to drop by more than half of their capacity after just a few hundred cycles, the new organic battery flaunts superior energy efficiency and life-expectancy related characteristics.
Since organic electrode materials are carbon, hydrogen, and oxygen that can be easily obtained through biomass, problems with the acquisition of resources are greatly reduced. Because it contains a very limited amount of transition metals, its price is very low and the production price can be adjusted according to process development, making it very easy to lower the production price.
Eco-friendly, low-cost organic electrode materials are a very important alternative, and the ability to solve the biggest technical challenges of new concepts like eco-friendly anode materials is of great significance to the scientific community.
Professor Kang commented on the research work as, "An exceptional research finding with the successful development and possibility of micro-pore control separation film material, which is encouraging in that organic secondary maps can be developed of energy storage devices that can be charged and discharged over a long cycle. It is expected to form a launch pad for future developments."
The research was conducted with support from the Ministry of Science and ICT and the Creative Materials Discovery Program through the National Research Foundation of Korea (NRF) under the Ministry of Science and ICT, and the results were published in the international journal of Nature Nanotechnology on November 2.
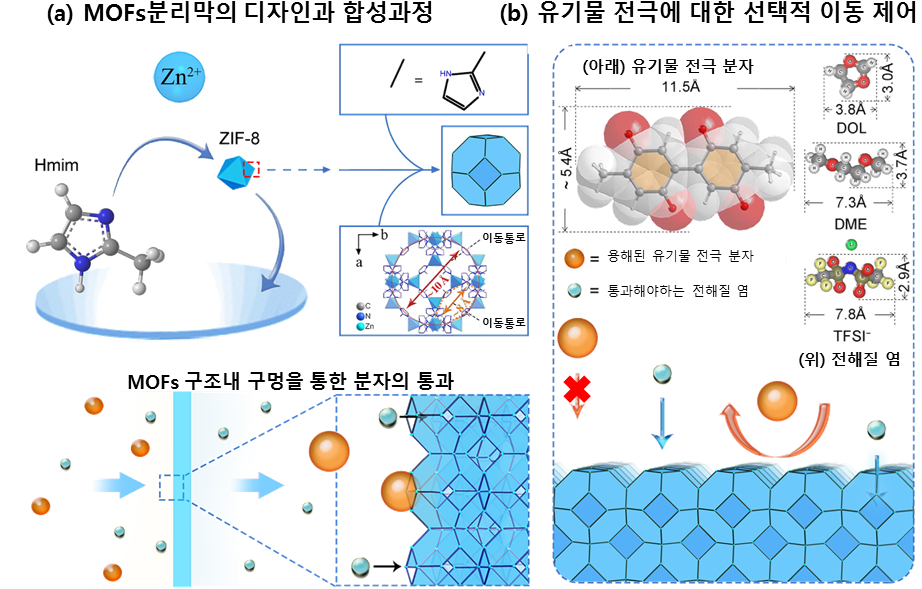
(a) A metal unit and organic unit were combined to design and synthesize an MOFs separator with nano-level movement passages. A sol-gel process has been introduced to successfully synthesize MOFs with uniform, dense, and shock-resistant holes.
(b) The holes in the designed MOFs separator are smaller than those of large organic electrode molecules and larger than those corresponding to small electrolyte salts. Thus, the dissolved organic electrodes can be prevented from moving to other electrodes while allowing the movement of molecules necessary for charging and discharging.
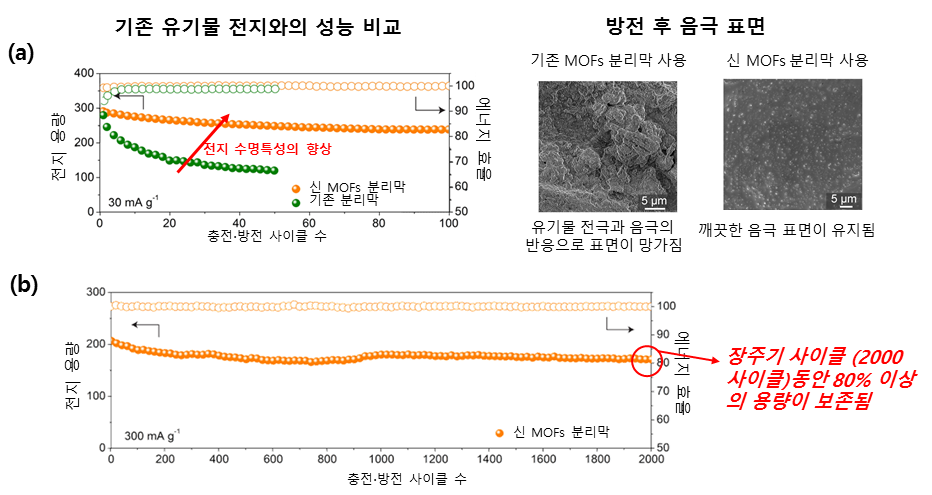
Picture 2. Dramatic improvement in organic secondary battery performance through the introduction of MOFs separation film.
(a) (Left) The introduction of manufactured MOFs separation film into organic secondary batteries has dramatically improved the life expectancy of the battery. In the case of using pre-existing separation membranes, the battery capacity was reduced by more than half (-43.0%) within 60 cycles, while the introduction of the new MOFs separation membranes preserved ~82.0% of the battery capacity for 100 cycles.
(Right) When observing the surface of cathodes after it is repeatedly charged and discharged, when the pre-existing separator was used, it was found that the transportation of organic charge particles and the cathode's reaction resulted in the molecules on the surface to be uneven. On the other hand, the introduction of MOFs separation membrane inhibited the movement of organic electrode molecules, maintaining a clean cathode surface.
(b) In the case of the introduction of the manufactured MOFs separator, it was confirmed that most battery capacities (~82.9%) were preserved, even after long cycles of charging and discharging that took place 2000 times. Here, the capacity reduction per cycle is only 0.008% and this is an amazing result that shows that long-term charging and discharging can be done in organic secondary batteries.