loaction
공대뉴스광장
SNU Professor Do Heui Kim's Team Finds an Easy and Effective Fine Dust Reduction Technology
-
작성자
관리자
-
등록일
2021.03.09
-
조회수
845
SNU Professor Do Heui Kim's Team Finds an Easy and Effective Fine Dust Reduction Technology
- After the large-scale verification process, POSCO Gwangyang Steelworks is successfully being commercialized
- Reduction in the industrial emission of fine dust, published in <Nature Communications> on February 10
- Reduction in the industrial emission of fine dust, published in <Nature Communications> on February 10

▲ (From left) SNU Professor Do Heui Kim of the Department of Chemical and Biological Engineering, Dr. Inhak Song, Dr. Hwangho Lee
A source proprietary technology that can effectively remove nitrogen oxides, an environmental pollutant that is known to be the cause of fine dust, was developed by Professor Do Heui Kim's Team of the Department of Chemical and Biological Engineering at Seoul National University's College of Engineering.
Through a industry-academia joint research with the Pohang Research Institute of Industrial Science & Technology (RIST) Fine Dust Research Center, Professor Do Heui Kim's research team developed a vanadium-based catalyst system that can reliably remove nitrogen oxides at low temperatures. With Dr. Inhak Song and Dr. Hwangho Lee as co-first-authors, the results of the study was published online as of February 10 in the international journal <Nature Communications> . (Title of the Research Paper: Simple physical mixing of zeolite prevents sulfur deactivation of vanadia catalysts for NOx removal)
Nitrogen (N2) and oxygen (O2) of high temperatures from combustion facilities such as power plants, incinerators, and steel mills react with each other to produce nitrogen oxides (NO, NO2). Nitrogen oxide is a well-known environmental pollutant that causes photochemical smog, acid rain and fine dust, and is known to irritate skin tissues and respiratory organs causing fatal diseases and many countries around the world, including Korea, are regulating its emissions. In particular, as nitrogen oxides in the atmosphere have recently been reported as the main cause of the generation of fine dust, regulations on nitrogen oxide emissions have been increasingly tightened.
Industrial combustion facilities are currently using selective catalytic reduction (SCR) technology through the use of ammonia (NH3) to remove nitrogen oxides. The system is a technology that converts nitrogen oxides into nitrogen that is harmless to the human body which is achieved through catalysts that use ammonia as a reducing agent. Metallic oxide catalysts that contain titanium oxide (TiO2) and small amounts of vanadium oxide (V2O5) are most commonly used. Vanadium catalysts are widely used because, unlike other catalysts like that of copper (Cu) and manganese (Mn), they can reliably remove nitrogen oxides without performance degradation by sulfur dioxide (SO2) that are contained in combustion emissions in areas with temperatures in the range of 300 to 400℃.
Recently, the growing demand for energy conservation in all industries has raised the need for SCR catalysts that can be operated at not just high temperature areas of 300 to 400℃ but also in low temperatures that are below 250℃.
However, at temperatures that are lower than 250℃, sulfur dioxide (SO2) contained in the gas is converted into the form of highly viscous ammonium bisulfate (ABS) and is deposited on the surface of the catalyst, resulting in a new problem that gradually reduces the catalyst's ability to reduce nitrogen oxides. Therefore, in order to successfully utilize SCR technology using vanadium catalysts even in low temperature conditions, the problem of reduced catalytic activity by ammonium bisulfate must be solved.
The research team was the first to discover that physically mixing zeolites, a porous substance used as adsorbents or catalysts, with conventional vanadium catalysts can selectively absorb ammonium bisulfate deposited on the catalyst's surface to suppress catalytic activity.
Based on this observation, the team proposed a new physical hybrid catalyst that protects the activity point of the vanadium catalyst by immediately absorbing ammonium bisulfate deposited in the vanadium catalyst at a low temperature of 220°C.
Through a joint study with Professor Jeong Woo Han of the Department of Chemical Engineering at Pohang University of Science and Technology, the team was also able to theoretically establish that zeolite's special structure has the effect of stabilizing ammonium bisulfate molecules under reaction conditions.
The source proprietary technology was able to be commercialized faster than other existing technologies due to the fact that the method of catalyst manufacturing was very simple and showed excellent performance improvement. In fact, the new catalyst that was developed has completed its large-scale verification process at the pilot plant, and is currently commercialized and successfully operated at POSCO Gwangyang Steelworks.
"We solved the problem of performance degradation of vanadium catalysts caused by sulfur in a simple and inexpensive way of physically mixing zeolite catalysts. The research results that theoretically and empirically explain the excellence of the new catalyst are also important, but the commercialization of a catalyst that was developed in a university hold much more meaning," said Professor Do Heui Kim.
The study was conducted with support from POSCO and the Ministry of Science and ICT's leading research center project (Korea University's
Super Ultra Low Energy and Emission Vehicle).
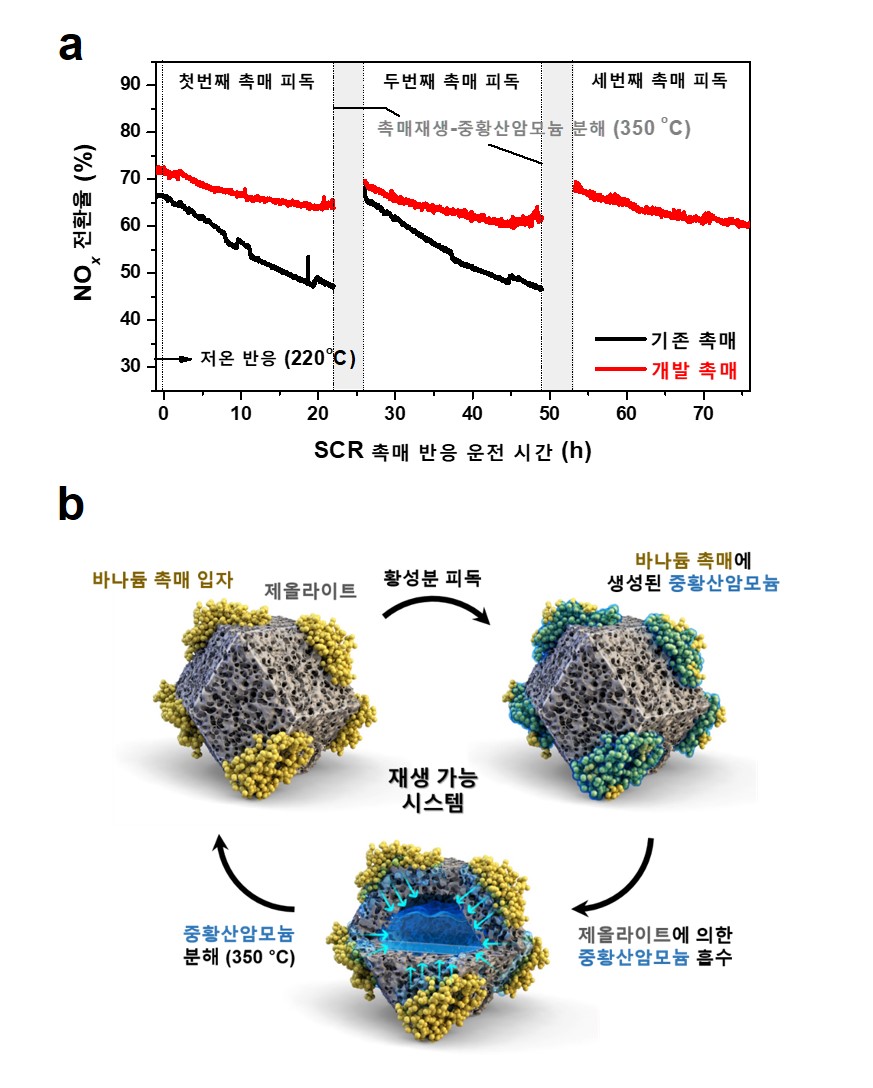
▲ Picture 1: (a) Comparison of the denitrification reaction in the conventional catalyst and the newly developed catalyst in Low Temperature (220℃) (b) Diagram summarizing the principles of operation of the developed low temperature catalyst.
담당부서대외협력실
전화번호880-9148