- About
- Academics
-
Undergraduate Programs
- Civil and Environmental Engineering
- Architecture and Architectural Engineering
- Mechanical Engineering
- Industrial Engineering
- Energy Resources Engineering
- Nuclear Engineering
- Materials Science and Engineering
- Electrical and Computer Engineering
- Naval Architecture and Ocean Engineering
- Computer Science and Engineering
- Aerospace Engineering
- Chemical and Biological Engineering
-
Graduate Programs
- Civil and Environmental Engineering
- Architecture and Architectural Engineering
- Mechanical Engineering
- Industrial Engineering
- Energy Systems Engineering
- Materials Science and Engineering
- Electrical and Computer Engineering
- Naval Architecture and Ocean Engineering
- Computer Science and Engineering
- Chemical and Biological Engineering
- Aerospace Engineering
- Interdisciplinary Program in Technology, Management, Economics and Policy
- Interdisciplinary Program in Urban Design
- Interdisciplinary Program in Bioengineering
- Interdisciplinary Program in Artificial Intelligence
- Interdisciplinary Program in Intelligent Space and Aerospace Systems
- Chemical Convergence for Energy and Environment Major
- Multiscale Mechanics Design Major
- Hybrid Materials Major
- Double Major Program
- Open Programs
-
Undergraduate Programs
- Research
- Campus Life
- Communication
- Prospective Students
- International Office
News
Professor Jeyong Yoon’s Research Team at SNU Develops Long-lasting Water Electrolysis Operation Technology Without Pre-synthesized Catalysts
-
Uploaded by
대외협력실
-
Upload Date
2025.05.29
-
Views
316
Professor Jeyong Yoon’s Research Team at SNU Develops Long-lasting Water Electrolysis Operation Technology Without Pre-synthesized Catalysts
- Published in the prestigious journal Nature Communications
- Proposes a strategy to replace expensive catalysts and maximize water electrolysis efficiency
- Expected to contribute to carbon neutrality and enhance Korea’s hydrogen production competitiveness
▲ (From left) Dr. Sanghwi Han, Department of Chemical and Biological Engineering, Seoul National University (first author); Assistant Professor Jang Yong Lee, Department of Chemical Engineering, Konkuk University (corresponding author); Assistant Professor Jaeyune Ryu, Department of Chemical and Biological Engineering, Seoul National University (corresponding author); Professor Jeyong Yoon, Department of Chemical and Biological Engineering, Seoul National University (corresponding author)
Seoul National University College of Engineering announced that the research team of Professors Jeyong Yoon and Jaeyune Ryu from the Department of Chemical and Biological Engineering, in collaboration with Professor Jang Yong Lee’s team from Konkuk University’s Department of Chemical Engineering, has developed a new water electrolysis operation strategy that can produce green hydrogen without complex catalyst manufacturing processes.
The research team presented the possibility of significantly increasing hydrogen production efficiency without the use of precious metal-based catalysts. As a result, this research outcome is expected to be a technological turning point that accelerates the realization of a carbon-neutral society.
These findings were published on May 23th in the world-renowned journal Nature Communications under the title “Dynamic polarization control of Ni electrodes for sustainable and scalable water electrolysis under alkaline conditions.”
Water electrolysis technology, which produces green hydrogen using electricity, is one of the core technologies for achieving carbon neutrality and is designated as one of Korea’s 12 national strategic technologies. Although electrolysis is gaining attention as a core method for eco-friendly hydrogen production, current systems require highly active catalyst layers to be precisely synthesized and applied to the electrode surfaces. These catalyst layers gradually degrade over time, presenting structural limitations.
To address this, the research team independently developed a novel “Electrochemical Activation (EA) operation” method. This method allows for high-efficiency and long-lasting hydrogen production using only commercial nickel (Ni) electrodes—eliminating the need for expensive, precision-engineered catalyst layers.
The research team applied their self-developed EA operation method to commercial Ni electrodes without any catalyst coating. As a result, they achieved water electrolysis efficiency comparable to that of high-performance Ni-Fe oxyhydroxide (NiFeOOH) catalysts, which are known for their excellence in the oxygen evolution reaction (OER)—the rate-determining step of water electrolysis.
The core technique lies in a method called Dynamic Polarization Control, which periodically gives the electrode a brief “rest.” By applying a weak reducing voltage to the Ni electrode for a short period, trace amounts of Fe dissolved in the KOH electrolytes are induced to reattach to the electrode surface. This reattached iron then bonds with the Ni to autonomously form a highly active oxygen evolution catalyst layer on the electrode. Through repeated cycles, the electrode maintains its activity and becomes a self-healing system.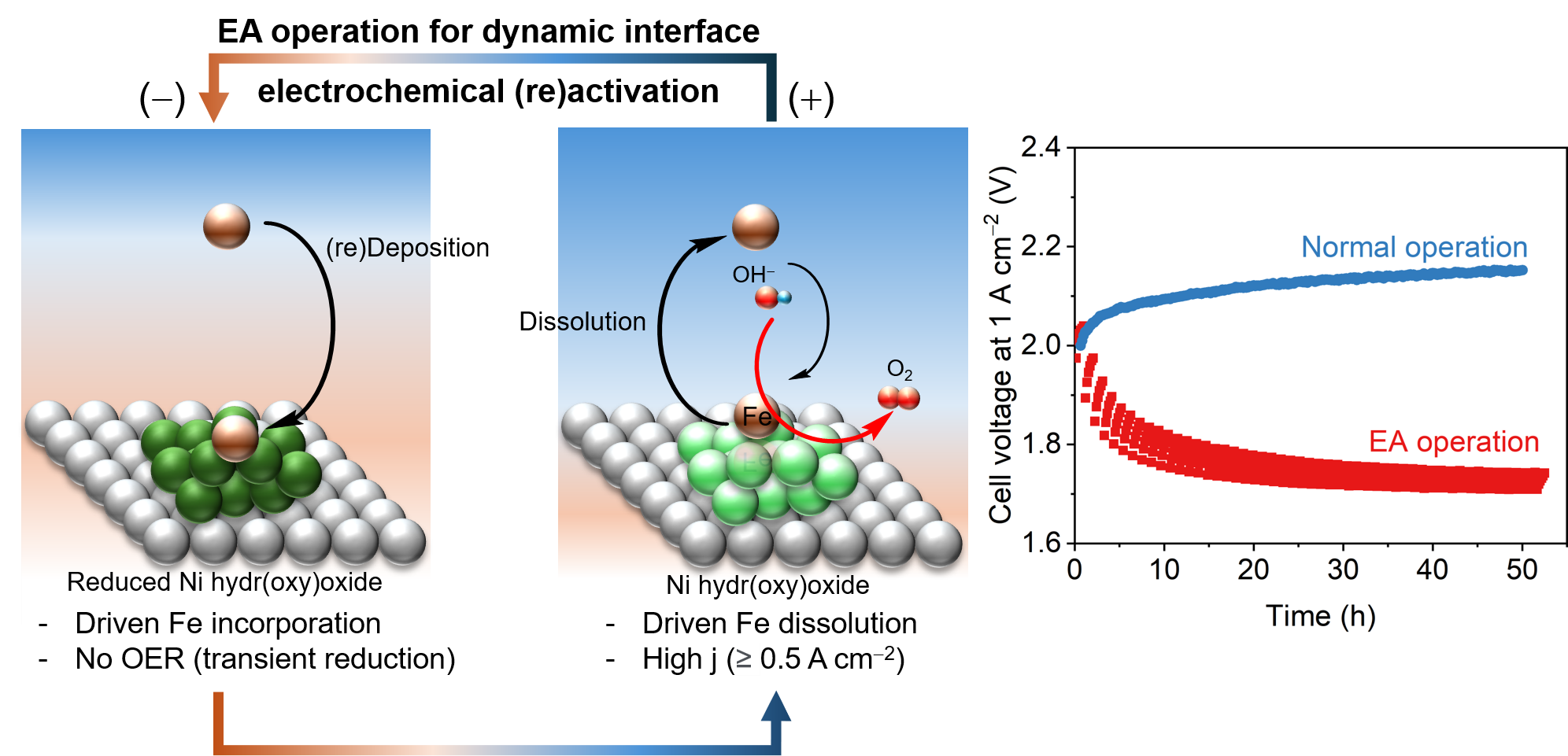
▲ Mechanism of the EA operation method developed by the research team (left), and durability comparison between conventional water electrolysis operation and the EA operation (right)
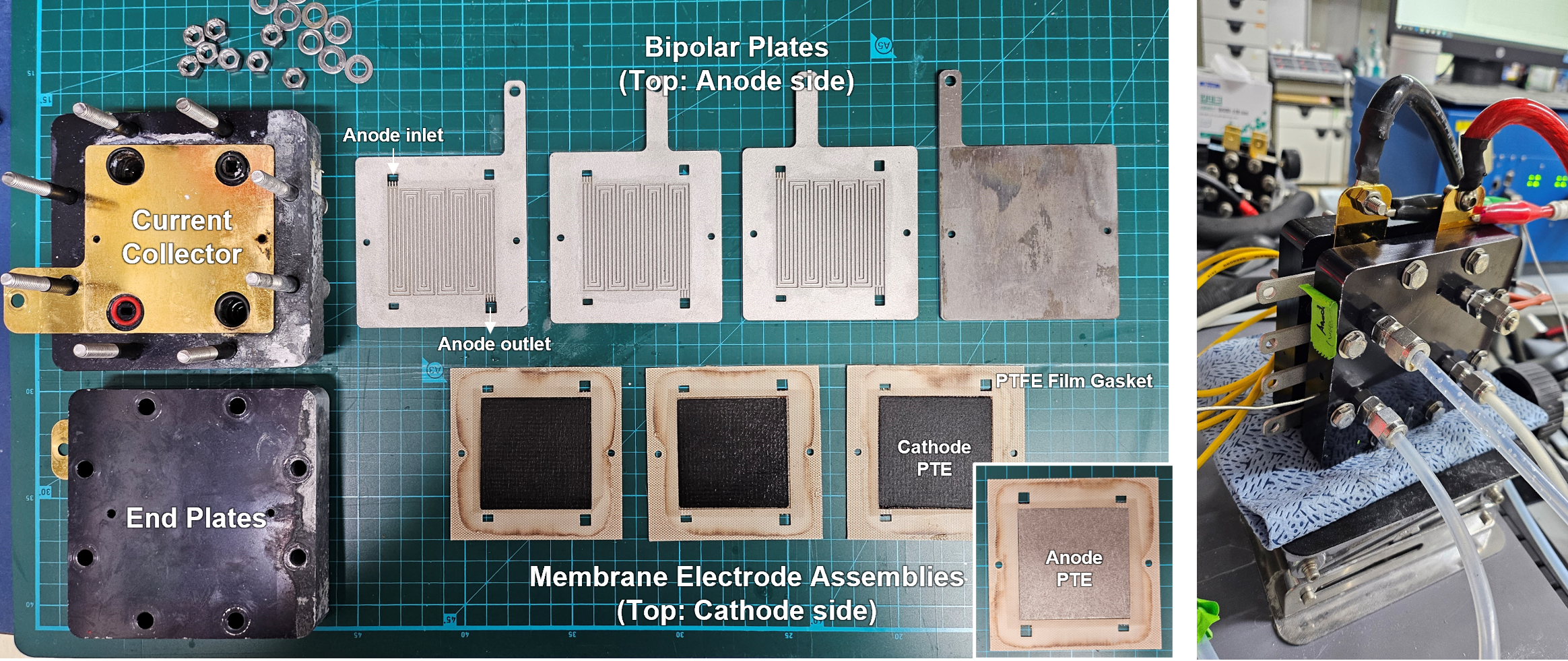
▲ Components of a 3-stack water electrolysis cell with 25 cm² area (left), and image of the system in operation (right)
A water electrolysis cell using this operation method operated stably for over 1,000 hours under high current density of 1 A/cm². Furthermore, the system demonstrated outstanding durability in a scaled-up configuration: a three-stack water electrolysis cell with a 25 cm² active area per cell also ran for several hundred hours. This result verifies the reliability of the technology not only at the lab scale, but also under realistic conditions involving extended operation and large-area systems.
The EA operation demonstrates the potential to significantly improve the economic feasibility of green hydrogen production by replacing costly precious metal catalysts and simplifying the hydrogen generation process. This method achieves both high efficiency and long-term stability without the need for expensive materials or complex manufacturing processes. It not only helps reduce the actual cost of hydrogen production but also exhibits strong reproducibility and scalability, making it highly promising for expansion to large-scale systems and commercial implementation.
Accordingly, this technology is expected to enhance the competitiveness of Korea’s hydrogen production processes through future technology transfer and industrial application. It is also anticipated to serve as a foundational technology that will support Korea’s realization of carbon neutrality and its transition to a hydrogen-based economy.
Professor Jeyong Yoon, who led the research, emphasized, “A hydrogen production method that does not rely on catalysts is a transformative strategy that can greatly improve the economics and scalability of green hydrogen. This achievement marks a real turning point for hydrogen economy technologies aimed at carbon neutrality.”
Co-leading the study, Professor Jaeyune Ryu added, “This research is not just about optimizing operating conditions. It is a fundamental application study that systematically clarified the complex electrochemical interactions at the electrode/electrolyte interface and successfully implemented them in a real system. It is a case where both theoretical insight and industrial relevance were effectively demonstrated.”
Dr. Sanghwi Han, the first author of this study from Seoul National University’s Department of Chemical and Biological Engineering, aspires to contribute to the global development of carbon-neutral technologies through electrochemical energy conversion research. Beginning in September, he will work as a postdoctoral researcher at the University of California, Berkeley, continuing his research activities in a world-class academic environment to further advance Korea’s leading technological capabilities. In the long term, Dr. Han plans to pursue a faculty position in Korea and play a pivotal role in the academic and industrial advancement of energy and environmental technologies.
This research was supported by several programs from National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2024-00449579), the NFR grant funded by the Nano & Material Technology Development Program by the Ministry of Science and ICT(RS-2024-00409675), the NRF grant funded by the MSIT (RS-2024-00348577, RS-2024-00415940, and RS-2024-00421181), and Institute for Basic Science (IBS-R006-D1).
[Reference Materials]
- Title/Journal : “Dynamic polarization control of Ni electrodes for sustainable and scalable water electrolysis under alkaline conditions”, Nature Communications
- DOI : https://doi.org/10.1038/s41467-025-60201-w
[Contact Information]
Dr. Sanghwi Han, Researcher at Professor Jeyong Yoon’s Laboratory, Department of Chemical and Biological Engineering, Seoul National University / hanhwi04@snu.ac.kr