- About
- Academics
-
Undergraduate Programs
- Civil and Environmental Engineering
- Architecture and Architectural Engineering
- Mechanical Engineering
- Industrial Engineering
- Energy Resources Engineering
- Nuclear Engineering
- Materials Science and Engineering
- Electrical and Computer Engineering
- Naval Architecture and Ocean Engineering
- Computer Science and Engineering
- Aerospace Engineering
- Chemical and Biological Engineering
-
Graduate Programs
- Civil and Environmental Engineering
- Architecture and Architectural Engineering
- Mechanical Engineering
- Industrial Engineering
- Energy Systems Engineering
- Materials Science and Engineering
- Electrical and Computer Engineering
- Naval Architecture and Ocean Engineering
- Computer Science and Engineering
- Chemical and Biological Engineering
- Aerospace Engineering
- Interdisciplinary Program in Technology, Management, Economics and Policy
- Interdisciplinary Program in Urban Design
- Interdisciplinary Program in Bioengineering
- Interdisciplinary Program in Artificial Intelligence
- Interdisciplinary Program in Intelligent Space and Aerospace Systems
- Chemical Convergence for Energy and Environment Major
- Multiscale Mechanics Design Major
- Hybrid Materials Major
- Double Degree Program
- Open Programs
-
Undergraduate Programs
- Campus Life
- Communication
- Prospective Students
- International Office
News
Research Team of Professors Nathaniel S. Hwang and Byung Gee Kim of SNU College of Engineering and Professor Dong Yun Lee of Hanyang University Develop 'Cell-Caging' Technology Through Nanofilm Formation.
-
Uploaded by
관리자
-
Upload Date
2021.07.05
-
Views
150
Research Team of Professors
Nathaniel S. Hwang and Byung Gee Kim of SNU College of Engineering and Professor Dong Yun Lee of Hanyang University
Develop 'Cell-Caging' Technology Through Nanofilm Formation
Nathaniel S. Hwang and Byung Gee Kim of SNU College of Engineering and Professor Dong Yun Lee of Hanyang University
Develop 'Cell-Caging' Technology Through Nanofilm Formation
- Opened the possibility of safely transplanting heterogeneous organs without immunosuppressants.
- Used electrostatic attraction to stack biomolecules in order of chitosan and hyaluronic acid
- Used electrostatic attraction to stack biomolecules in order of chitosan and hyaluronic acid

▲ (From left) Professor Nathaniel S. Hwang, Professor Byung Gee Kim, Professor Dong Yun Lee
Seoul National University College of Engineering (Dean Kookheon Char) announced on June 24 that a research team led by Professor Nathaniel S. Hwang and Professor Byung Gee Kim of the Department of Chemical and Biological Engineering and Professor Dong Yun Lee of Hanyang University's Department of Bioengineering has developed "cell caging" technology that forms a nanofilm on the cell surface using biopolymer crosslinking technology.
'Cell Caging' technology prevents immune rejection when transplanting heterogeneous pancreatic cells and allows smooth insulin secretion of cells, opening the possibility of treating type-1 diabetes patients without immunosuppressants.
The research team succeeded in producing nanofilms by sequentially stacking biopolymers of chitosan and hyaluronic acid, using electrostatic attraction. In order to overcome the problem of low sustainability of the existing nanofilm stacking method, a cross-linking method using the enzyme that was newly developed by the joint research team, S.av. tyrosinase, was used which has produced stronger and more sustainable films. The newly developed tyrosinase has a significantly faster cross-linking speed compared to conventional enzymes, making it useful to be applied to clinical sites.
The nanofilm that has had the application of the enzyme cross-linking method has a relatively thin thickness of about 140 nm, but it is capable of maintaining stability against physical stimuli and protects cells from immune cell attacks.
Previously, alginate was popularly used as a material for cell therapy transplantation for type-1 diabetes treatment.
However, the thickness of the capsule makes blood sugar recognition-insulin secretion less immediate and makes it inherent to the risk of fibrosis caused by an immune response induced by the alginic acid itself. The nanofilm technology developed by the research team allows immediate blood sugar recognition and insulin secretion due to its thinness.
The research team applied the caging technology to the mouse's pancreatic cells, MIN6 cells, and successfully controlled blood sugar by implanting it into the kidney of the mouse that caused type-1 diabetes. Compared to groups that did not introduce cross-linking technology, blood sugar control in the bridged nanofilm group was smooth, proving the superiority of enzyme cross-linking.
Further, nanofilm technology can be applied to both single cells and spheroids, which can be applied to heterogeneous organ transplantation and disease treatment using stem cells.
"Using the ‘cell cage’ technology developed in this study, it is expected that the immune rejection response, the biggest problem in the treatment of type 1 diabetes through beta cell transplantation, will be overcome. Our goal is to accelerate the practical use of 'cell-caging' technology through continuous research and development," said Professor Nathaniel S. Hwang.
The research team, along with international patent applications for their 'Cell Caging' technology, was selected as a preliminary start-up team for the 'Laboratory-specific Startup Leading University Project' (National Research Foundation of Korea) and the 'Bio Promising Technology Global Startup Support Project'
(Bio I-Corps) and announced their plans to cooperate with domestic and foreign cell therapy development companies in the future.
Meanwhile, the findings were published online on June 23 in the international journal 'Science Advances'. (Research Paper Title: Novel Enzymatic Crosslinking-based Hydrogel Nanofilm Caging System on Pancreatic β-cell Spheroid for Long-term Blood Glucose Regulation)
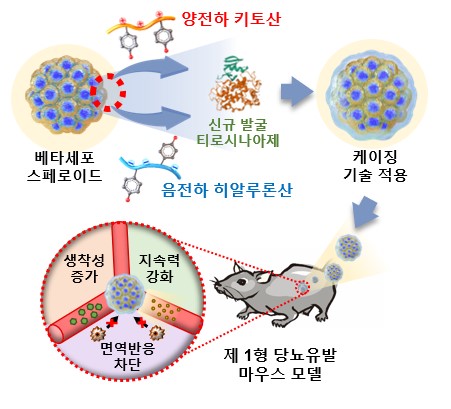
▲ Diagram of the cell caging technology and diabetes-induced mouse model transplantation process
developed by the research team