- About
- Academics
-
Undergraduate Programs
- Civil and Environmental Engineering
- Architecture and Architectural Engineering
- Mechanical Engineering
- Industrial Engineering
- Energy Resources Engineering
- Nuclear Engineering
- Materials Science and Engineering
- Electrical and Computer Engineering
- Naval Architecture and Ocean Engineering
- Computer Science and Engineering
- Aerospace Engineering
- Chemical and Biological Engineering
-
Graduate Programs
- Civil and Environmental Engineering
- Architecture and Architectural Engineering
- Mechanical Engineering
- Industrial Engineering
- Energy Systems Engineering
- Materials Science and Engineering
- Electrical and Computer Engineering
- Naval Architecture and Ocean Engineering
- Computer Science and Engineering
- Chemical and Biological Engineering
- Aerospace Engineering
- Interdisciplinary Program in Technology, Management, Economics and Policy
- Interdisciplinary Program in Urban Design
- Interdisciplinary Program in Bioengineering
- Interdisciplinary Program in Artificial Intelligence
- Interdisciplinary Program in Intelligent Space and Aerospace Systems
- Chemical Convergence for Energy and Environment Major
- Multiscale Mechanics Design Major
- Hybrid Materials Major
- Double Major Program
- Open Programs
-
Undergraduate Programs
- Research
- Campus Life
- Communication
- Prospective Students
- International Office
News
Professor Ki Tae Nam's Team of SNU College of Engineering Produces the World's First Carbon-Neutral Fuel That Mimics the Natural Biofuel Synthesis System
-
Uploaded by
관리자
-
Upload Date
2021.07.20
-
Views
910
Professor Ki Tae Nam's Team of SNU College of Engineering Produces the World's First Carbon-Neutral Fuel That Mimics the Natural Biofuel Synthesis System
- Development of high-efficiency carbon dioxide conversion system by
applying the principles of biofuel synthesis
- Expected to propose a new paradigm for electrochemical carbon dioxide conversion technology

▲ Professor Ki Tae Nam's research team of Seoul National University College of Engineering's Department of Materials Science and Engineering: (From left) Researchers Jun Ho Jang, Kyu Min Lee and Professor Ki Tae Nam
Seoul National University College of Engineering (Dean Kookheon Char) announced on July 9 (Friday) that Professor Ki Tae Nam's research team of the Department of Materials Science and Engineering developed the world's first electrochemical carbon dioxide conversion technology that mimics the natural biofuel synthesis system and succeeded in synthesizing carbonate, a new concept of e-fuel (e-fuel) from carbon dioxide.
Professor Ki Tae Nam's research team of the SNU Department of Materials Science and Engineering succeeded in establishing a new electrochemical carbon dioxide conversion system that has never been proposed before by applying the core principle that is used in nature to synthesize biofuels to electrochemical carbon dioxide conversion. Through this, it is expected to present a new paradigm in the field of carbon dioxide conversion and utilization by developing a highly efficient system that can produce high value-added products with only a small electrical energy cost.
Reducing the concentration of greenhouse gases in the atmosphere is currently a top priority for mankind. Countries around the world are tightening carbon regulations along with the declaration of 2050 Carbon Neutrality Coalition and in order to implement carbon neutrality, it is not only the efforts to reduce carbon emissions that are essential, but also the technology to convert emitted carbon dioxide into high-value-added compounds.
As global responses to reduce greenhouse gas emissions is in full swing, 'e-fuel', a carbon neutral fuel, has recently been in the limelight. ‘e-fuel’ is a technology that converts carbon dioxide into high value-added compounds by using electric energy. By utilizing eco-friendly energy such as solar and wind power as its energy source to reduce greenhouse gases and produce useful products, it is expected to be a core technology that can implement a carbon neutral society.
The technological principle of producing ‘e-fuel’ from existing carbon dioxide is as follows. To provide an example, when we burn gasoline, gasoline molecules oxidize and turn into carbon dioxide, which powers a car's engine. Conversely, when carbon dioxide is reduced using electrical energy, electrons are injected into the carbon dioxide and converted into high energy fuels such as gasoline. Until now, through this reduction process alone, electrochemical carbon dioxide conversion studies have produced materials such as carbon monoxide, formic acid and ethylene, which have higher energy than carbon dioxide. However, this method has the limitation of more electricity being generated in order to obtain a material with high energy. Therefore, for the commercialization of the electrochemical conversion technology of carbon dioxide, its low economic feasibility leaves the industry and academia to be looking for a new breakthrough.
Professor Ki Tae Nam's research team of Seoul National University's Department of Materials Science and Engineering presented a new breakthrough in 'e-fuel' production technology by applying the principle of synthesizing biofuels in the natural world to the electrochemical carbon dioxide conversion system. In the process of synthesizing biofuels, living organisms utilize the continuous electron transport flow created through the electron transport medium.
By mimicking the flow of such electrons, a new electrochemical carbon dioxide conversion system has been developed that does not just end with electrons being injected into carbon dioxide, but can escape again through the electron transport medium and flow continuously through the solution. As a result, a new methodology, completely different from those of conventional methods, was proposed for reducing carbon dioxide and it made possible the synthesis of carbonate compounds from carbon dioxide, which was thought to be impossible until now. This is significant in that it has expanded the range of ‘e-fuel’ products that can be produced from carbon dioxide, which had previously been limited to products in reduced form.
The ‘e-fuel’ synthesis system is an innovative system that allows the formation of high-value compounds with low electrical energy costs. Dimethyl carbonate (hereinafter DMC), synthesized by Professor Ki Tae Nam's research team of the Seoul National University Department of Materials Science and Engineering, can be used as a fuel additive for gasoline and diesel, as well as being used in various industries such as polymer manufacturing, medicine and batteries. Due to such high utility value, the market price of DMC is about three times higher than that of formic acid that can be obtained through the existing carbon dioxide conversion.
On the other hand, the electrical energy required to produce DMC is 3.5 kWh/kg, which is similar to formic acid and considering that the unit cost of solar power generation in 2030 is 94.2 won/kWh, the research team can produce 1 kg of DMC with an electric energy cost of about 330 won. It is expected that it will be possible to take one step closer to the commercialization of electrochemical carbon dioxide conversion technology through new development capable of synthesizing products with high market price compared to low electrical energy.
"This achievement is innovative in that it suggested a method for synthesizing carbonate compounds from carbon dioxide at room temperature using electric energy for the first time in the world, and is expected to play an important role in carbon dioxide reduction technology. By developing a new concept of a carbon dioxide conversion system that enables the synthesis of high value-added compounds even with low electric energy cost, it is expected to present a new direction for electrochemical carbon dioxide conversion technology in the future,” said Professor Ki Tae Nam of Seoul National University's Department of Materials Science and Engineering.
Meanwhile, researchers Lee Kyu-min and Jang Joon-ho, the first authors of the study, are currently conducting master's and doctorate programs at Seoul National University, respectively.
This research was supported by the Future Material Discovery Project, the Mid-sized Researcher Support Project, the Nano Future Material Source Technology Development Project, and the Natural Description Innovation Technology Development Project.
The research was published online on July 9 in Nature Energy, a world-renowned international journal. Nature Energy is the world's most prestigious academic journal in energy, with the Impact Factor at 60.85 as of 2020.
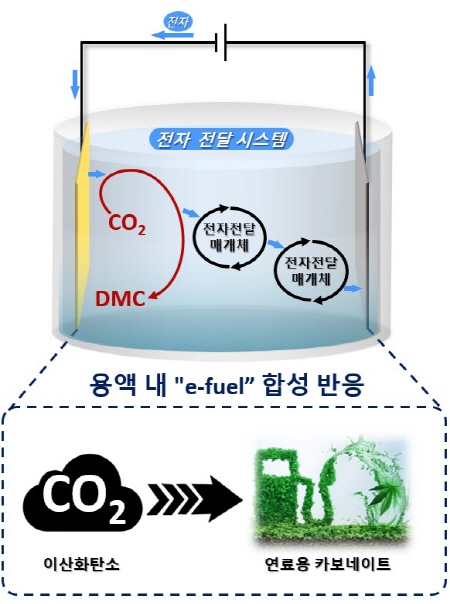
▲ Diagram showing the newly developed concept of carbon neutral fuel(e-fuel)'s synthesis reaction