- About
- Academics
-
Undergraduate Programs
- Civil and Environmental Engineering
- Architecture and Architectural Engineering
- Mechanical Engineering
- Industrial Engineering
- Energy Resources Engineering
- Nuclear Engineering
- Materials Science and Engineering
- Electrical and Computer Engineering
- Naval Architecture and Ocean Engineering
- Computer Science and Engineering
- Aerospace Engineering
- Chemical and Biological Engineering
-
Graduate Programs
- Civil and Environmental Engineering
- Architecture and Architectural Engineering
- Mechanical Engineering
- Industrial Engineering
- Energy Systems Engineering
- Materials Science and Engineering
- Electrical and Computer Engineering
- Naval Architecture and Ocean Engineering
- Computer Science and Engineering
- Chemical and Biological Engineering
- Aerospace Engineering
- Interdisciplinary Program in Technology, Management, Economics and Policy
- Interdisciplinary Program in Urban Design
- Interdisciplinary Program in Bioengineering
- Interdisciplinary Program in Artificial Intelligence
- Interdisciplinary Program in Intelligent Space and Aerospace Systems
- Chemical Convergence for Energy and Environment Major
- Multiscale Mechanics Design Major
- Hybrid Materials Major
- Double Major Program
- Open Programs
-
Undergraduate Programs
- Research
- Campus Life
- Communication
- Prospective Students
- International Office
News
SNU Materials Science and Engineering Team Identifies Reconstruction Mechanism of Copper Alloy Catalysts for CO₂ Conversion
-
Uploaded by
대외협력실
-
Upload Date
2025.08.12
-
Views
510
SNU Materials Science and Engineering Team Identifies Reconstruction Mechanism of Copper Alloy Catalysts for CO₂ Conversion
- Featured on the cover of Nature Catalysis, the field’s leading journal
- Breakthrough in overcoming CO₂ conversion catalyst challenges for carbon neutrality
▲ Top row (from left): Prof. Young-Chang Joo (Department of Materials Science and Engineering, Seoul National University), Prof. Dae-Hyun Nam (Department of Materials Science and Engineering, Korea University), Prof. Jungwon Park (Department of Chemical and Biological Engineering, Seoul National University), and Prof. Seoin Back (KU-KIST Graduate School of Converging Science and Technology, Korea University).
Bottom row (from left): Intae Kim (Department of Materials Science and Engineering, Seoul National University), Dr. Gi-Baek Lee (Department of Materials Science and Engineering, Seoul National University), Dr. Sungin Kim (Department of Chemical and Biological Engineering, Seoul National University), and Hyun Dong Jung (Department of Chemical and Biomolecular Engineering, Sogang University).
Seoul National University College of Engineering announced that a joint research team led by Professor Young-Chang Joo (Department of Materials Science and Engineering) and Professor Jungwon Park (School of Chemical and Biological Engineering) has, in collaboration with Professors Dae-Hyun Nam (Department of Materials Science and Engineering) and Seoin Baek (KU-KIST Graduate School) at Korea University, become the first in the world to elucidate the reconstruction mechanism of copper alloy catalysts during electrochemical CO₂ conversion reactions.
The research sheds light on atomic rearrangements in catalyst surface structures during reaction and presents a methodology for predicting and designing actual active sites in operando conditions. The findings were published in Nature Catalysis and selected as the cover article.
Electrochemical reduction of CO₂ has emerged as a pivotal technology in achieving carbon neutrality, enabling the transformation of greenhouse gas CO₂ into clean and valuable chemical feedstocks. Copper (Cu)-based catalysts are particularly notable for producing high-value multi-carbon compounds such as ethylene (C₂H₄) and ethanol (C₂H₅OH).
However, single-metal Cu catalysts face intrinsic limitations in selectively controlling the reaction pathways. Alloying Cu with other metals to create multiple active sites has been a strategy to enhance product selectivity and catalytic efficiency. While previous studies have focused on synthetic control of surface composition and nanostructure, they have overlooked dynamic changes under actual reaction conditions.
During CO₂ electroreduction, dynamic reconstruction of the catalyst surface—due to repeated metal dissolution and redeposition—becomes inevitable. This often disrupts the finely tuned surface structure originally designed for optimal activity, making it difficult to predict or optimize catalyst performance. The complexity is amplified in bimetallic or multimetallic systems, where the roles of different metal species in the reconstruction process remain largely unexplored. Therefore, understanding and controlling reconstruction phenomena in such systems is a critical step toward advancing CO₂ reduction catalyst design.
The researchers established a material selection map based on the oxophilicity and miscibility between Cu and the secondary metal X, and fabricated four representative Cu–X alloy catalysts (X = Ag, Fe, Zn, Pd). These catalysts were integrated into gas-diffusion electrodes and subjected to industrially relevant high-current CO₂ electroreduction conditions to induce surface reconstruction. Using cross-sectional transmission electron microscopy (TEM), they succeeded in capturing the surface structure changes—an achievement that overcomes the limitations of previous low-current-density catalyst reconstruction studies.
Notably, Cu–Ag catalysts exhibited surface formation of Cu nanoparticles during reaction, while Cu–Zn alloys maintained a uniform elemental distribution. Despite similar CO-producing capabilities, the reconstruction behavior yielded stark differences in product selectivity. In Cu–Ag, the Cu nanoparticles promoted further conversion of CO intermediates to ethanol, preserving high ethanol selectivity even at high Ag content. In contrast, Cu–Zn showed a decline in ethanol production due to a lack of Cu-rich active sites, favoring CO desorption instead.
Furthermore, through in-situ liquid-phase TEM, the researchers directly observed the nucleation and growth of Cu nanoparticles and identified a selective dissolution–redeposition mechanism induced by intermediate adsorption. They also demonstrated that the rearrangement behavior of redeposited atoms was determined by the miscibility of alloy components. Crucially, they applied a pulsed potential strategy to control dissolution–redeposition kinetics and successfully shifted product selectivity in Cu–Zn from CO toward ethanol.
This study provides a “design map” for understanding and predicting surface reconstruction in Cu-based bimetallic catalysts, offering a theoretical foundation for designing catalysts that dynamically adapt during operation. The design principles may be generalized to more complex multimetallic systems, ultimately advancing the commercialization of CO₂ conversion technologies by improving catalytic performance and durability.
Professor Young-Chang Joo remarked, “This is the first study to systematically unveil the dynamic reconstruction behavior of alloy catalysts during electrochemical CO₂ reduction. By moving beyond optimization of synthesis conditions and incorporating in-situ structural evolution into catalyst design, we present a new paradigm in high-performance catalyst development.”
Lead author Intae Kim, a combined Master-PhD student in the SNU Department of Materials Science and Engineering, plans to expand the framework of dynamic catalyst design by investigating the kinetics of reconstruction under pulsed CO₂ reduction conditions.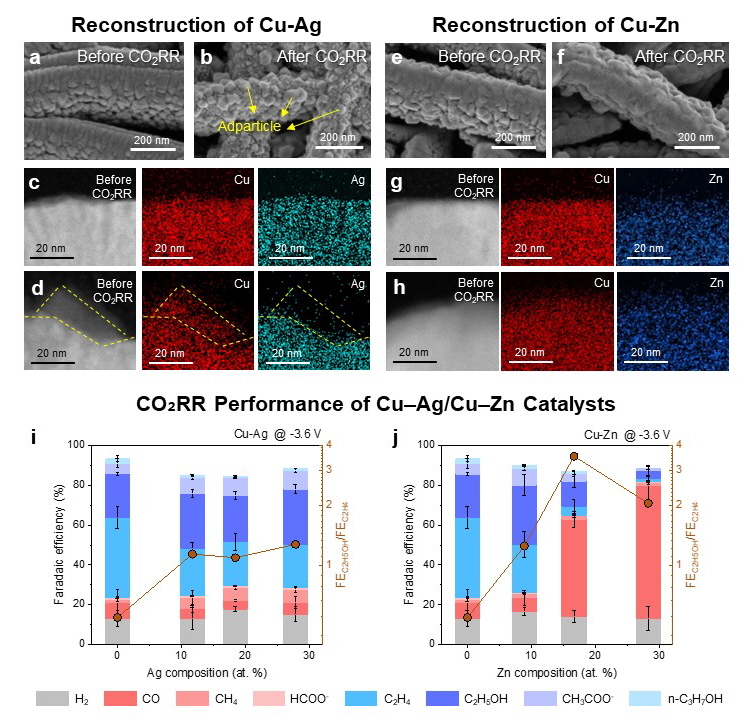
▲ Figure 1: a–d, Surface structure and elemental mapping of Cu–Ag alloy before and after CO₂RR.
e–h, Surface structure and mapping of Cu–Zn alloy before and after CO₂RR.
i–j, Product selectivity as a function of alloy composition.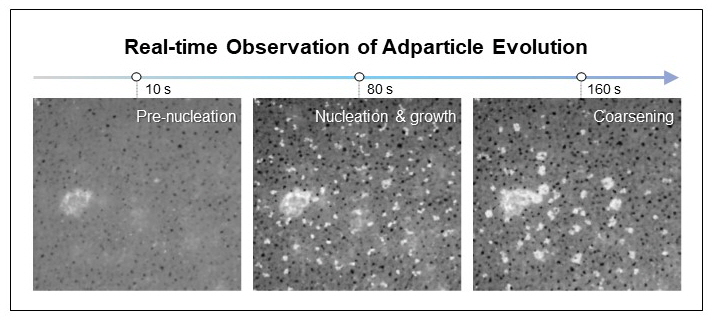
▲ Figure 2: In-situ liquid-phase TEM observation of nanoparticle formation and growth on Cu–Ag thin films.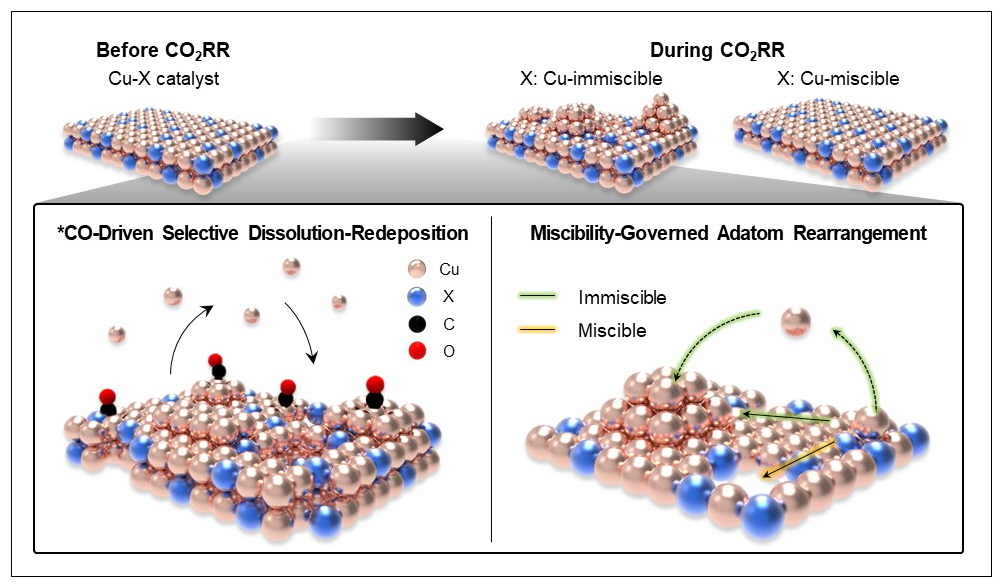
▲ Figure 3: Schematic of the reconstruction mechanism governed by intermediate adsorption and metal miscibility.
[Reference Materials]
- Title/Journal : “Unveiling the reconstruction of copper bimetallic catalysts during CO₂ electroreduction,” Nature Catalysis
- DOI : https://doi.org/10.1038/s41929-025-01368-9
[Contact Information]
Researcher In-Tae Kim, Nano Flexible Device Materials Lab, Department of Materials Science and Engineering, Seoul National University / +82-2-880-5817 / capintae@snu.ac.kr