- About
- Academics
-
Undergraduate Programs
- Civil and Environmental Engineering
- Architecture and Architectural Engineering
- Mechanical Engineering
- Industrial Engineering
- Energy Resources Engineering
- Nuclear Engineering
- Materials Science and Engineering
- Electrical and Computer Engineering
- Naval Architecture and Ocean Engineering
- Computer Science and Engineering
- Aerospace Engineering
- Chemical and Biological Engineering
-
Graduate Programs
- Civil and Environmental Engineering
- Architecture and Architectural Engineering
- Mechanical Engineering
- Industrial Engineering
- Energy Systems Engineering
- Materials Science and Engineering
- Electrical and Computer Engineering
- Naval Architecture and Ocean Engineering
- Computer Science and Engineering
- Chemical and Biological Engineering
- Aerospace Engineering
- Interdisciplinary Program in Technology, Management, Economics and Policy
- Interdisciplinary Program in Urban Design
- Interdisciplinary Program in Bioengineering
- Interdisciplinary Program in Artificial Intelligence
- Interdisciplinary Program in Intelligent Space and Aerospace Systems
- Chemical Convergence for Energy and Environment Major
- Multiscale Mechanics Design Major
- Hybrid Materials Major
- Double Major Program
- Open Programs
-
Undergraduate Programs
- Research
- Campus Life
- Communication
- Prospective Students
- International Office
News
SNU Researchers Solve Key Technical Challenge in Single-Crystalline Cathodes, Enabling Higher-Density, More Durable Batteries
-
Uploaded by
대외협력실
-
Upload Date
2026.01.08
-
Views
497
SNU Researchers Solve Key Technical Challenge in Single-Crystalline Cathodes, Enabling Higher-Density, More Durable Batteries
- Joint Work with SK On Demonstrates High-Density Single-Crystalline Cathodes with Large, ~10 μm Particles
- New Synthesis Pathway Proposed; Findings reported in Nature Energy
- Demonstrated ImprovedDurability, Safety-Related Metrics, and Volumetric Performance, with Scalability-Oriented Follow-Up Work Ongoing
▲ (From left) Prof. Kisuk Kang, Department of Materials Science and Engineering, Seoul National University; and Youngjun Jeon, Researcher, Department of Materials Science and Engineering, Seoul National University
Seoul National University College of Engineering reports that a research team led by Prof. Kisuk Kang of the Department of Materials Science and Engineering has successfully developed high-density single-crystalline cathode electrodes composed of large particles through joint research with SK On.
This study resolves long-standing technical challenges in the synthesis of single-crystal cathode materials and presents a new synthesis route. The work identifies a longstanding synthesis bottleneck in single-crystalline cathodes and reports a new route, published in Nature Energy.
Polycrystalline cathode materials, which are widely used in the battery industry today, consist of agglomerates of multiple primary particles. During calendering processes or repeated charge–discharge cycling, these structures are prone to cracking, which can accelerate degradation and increase gas evolution under harsh operating conditions. In contrast, single-crystalline cathode materials, in which each particle consists of a single continuous crystal structure, are far more resistant to cracking and therefore offer superior lifetime and stability.
Despite these advantages, synthesizing single-crystal cathodes with both large and uniform particle sizes while maintaining structural stability has been considered a major technical challenge. In particular, cathode materials with high nickel content require high-temperature and long-duration heat treatments to form single crystals. During this process, however, cation disorder* tends to occur, leading to degraded battery performance and shortened cycle life.
* Cation disorder: A phenomenon in nickel-based cathode materials in which lithium and nickel ions—having similar ionic radii—deviate from their designated lattice layers and become intermixed. This disrupts lithium-ion transport, resulting in reduced power output and slower charge–discharge rates.
To overcome these limitations, the SNU research team and SK On devised a new synthesis strategy. The approach begins by first forming sodium-based single crystals, which exhibit high structural stability and favorable crystal growth characteristics**, and then converting them into lithium-based materials through an ion-exchange process. This method enables the preservation of a robust single-crystal framework while successfully producing lithium-based cathode materials.
** Crystal growth: The process by which atoms or ions arrange themselves in a regular lattice and gradually form a larger crystal.
The researchers also focused on large-particle single crystals, which are advantageous for achieving high energy density. They systematically analyzed optimal synthesis conditions—including chemical composition, temperature, and processing time—as well as the mechanisms governing structural formation. As a result, they successfully developed an ultrahigh-nickel*** single-crystalline cathode material with a particle size of 10 μm, comparable to the secondary particles of conventional polycrystalline cathodes, while completely eliminating cation disorder.
*** Ultrahigh nickel: Refers to cathode materials with a nickel content exceeding 94 percent. Higher nickel content directly translates into higher energy density, which increases the driving range of electric vehicles per charge.
The newly developed single-crystal cathode exhibited excellent mechanical and chemical stability, as well as high energy density. Experimental results confirmed that the absence of cation disorder significantly reduced structural distortion, while gas evolution was decreased by a factor of 25 compared to conventional polycrystalline cathodes. In addition, the electrode density reached 77 percent of the theoretical crystal density****.
**** Theoretical crystal density: The density of a perfectly ordered crystal lattice assumed to be free of defects and impurities.
Building on these findings, the SNU research team and SK On plan to continue follow-up studies aimed at developing next-generation cathode materials. In parallel, they are exploring more advanced material compositions and synthesis techniques, as well as strategies for maximizing energy density by combining single-crystalline particles of different sizes at optimized ratios.
Prof. Kisuk Kang stated, “This achievement addresses a key synthesis challenge for single-crystalline cathode materials and establishes an important foundation for next-generation battery technologies. We will continue to pursue innovative battery materials research through close collaboration with industry.”
Youngjun Jeon, a researcher in the Department of Materials Science and Engineering at SNU, added, “This study provided deeper insight into the growth mechanisms and structural stability of single-crystalline cathode materials. We hope that these results will contribute to improved battery performance and more efficient manufacturing processes, thereby supporting industrial advancement.”
Meanwhile, Youngjun Jeon is continuing follow-up research aimed at elucidating the crystal growth mechanisms of single-crystalline cathode materials. By achieving a more precise understanding of the underlying behavior, he plans to establish a foundation for expanding this work into a new synthesis paradigm, while simultaneously improving both material performance and manufacturing efficiency.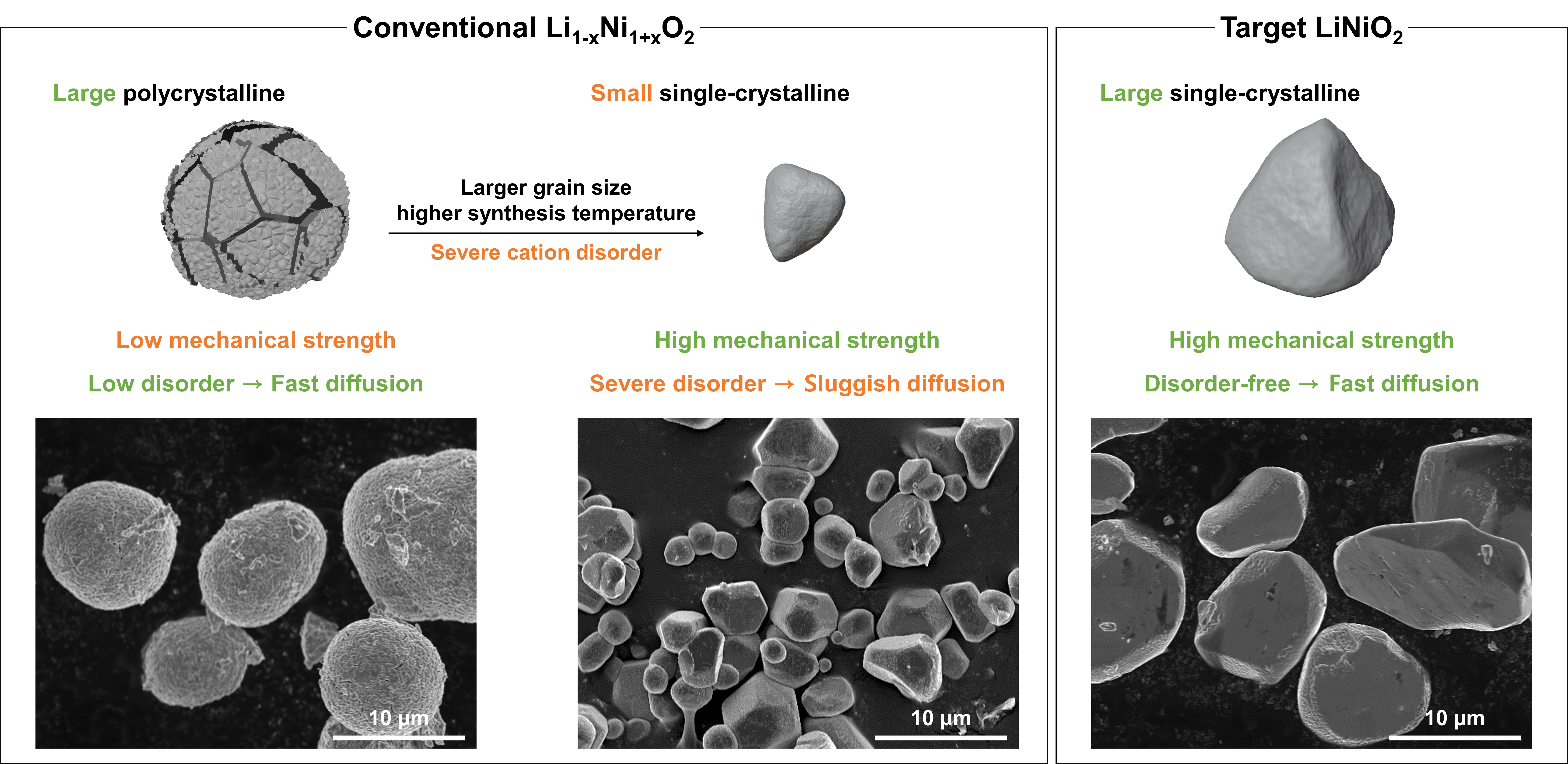
▲ Figure 1. Conventional LiNiO₂ cathode material (left) and desirable LiNiO₂ cathode material for high-energy-density electrode (right).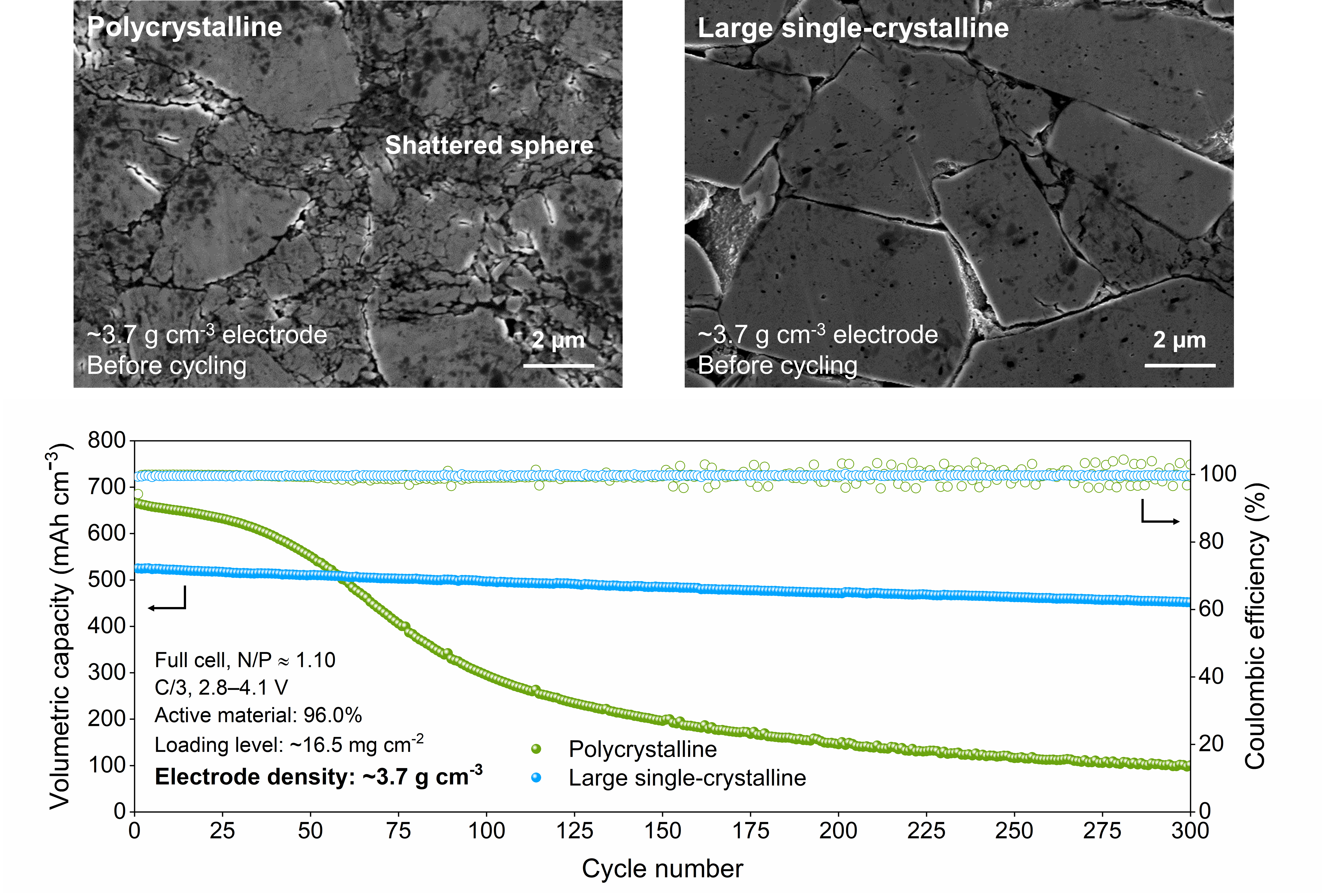
▲ Figure 2. State of the active material after high-density electrode fabrication (top) and full-cell cycle performance (bottom).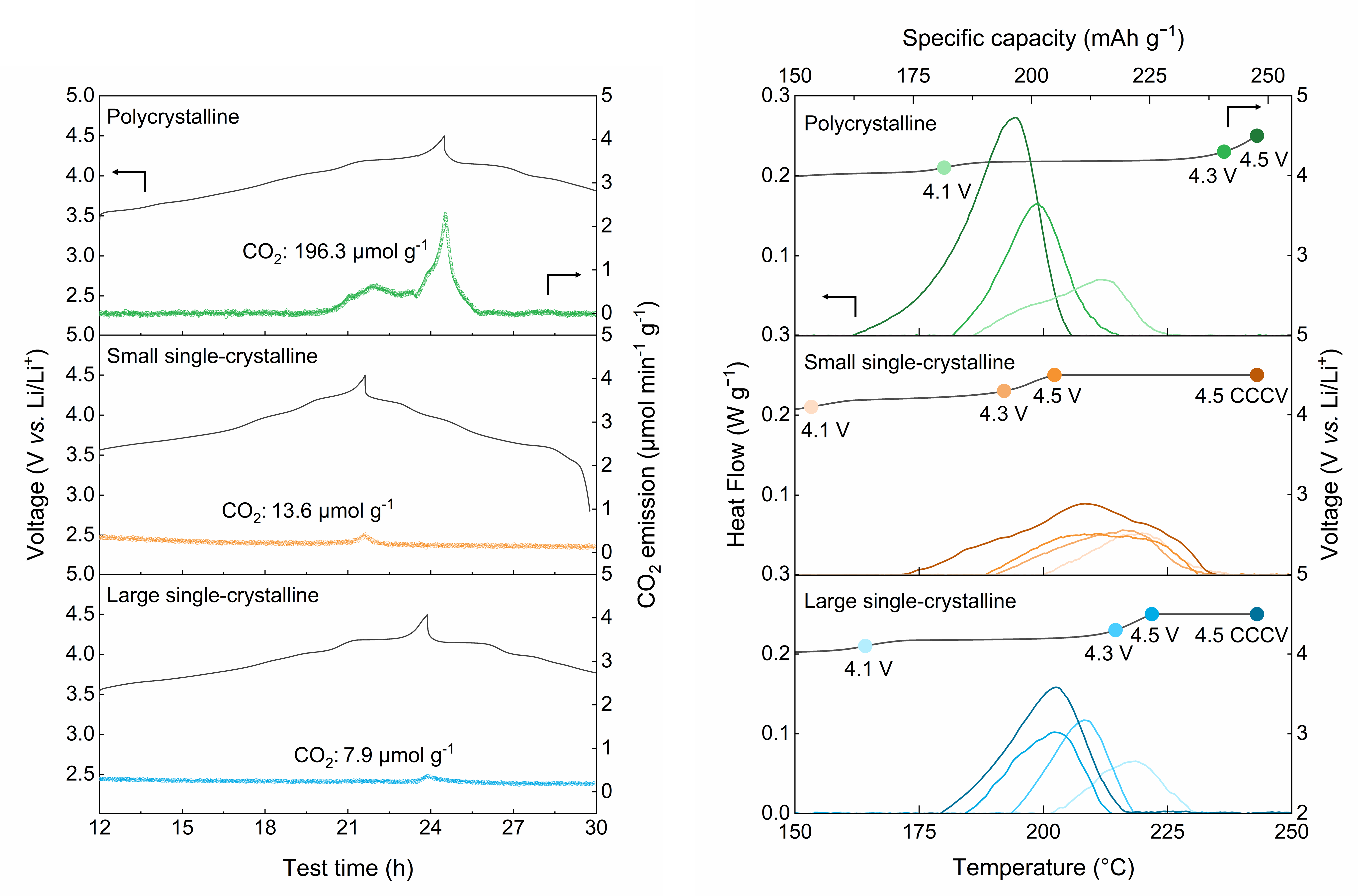
▲ Figure 3. Battery safety evaluation results, including gas evolution analysis (left) and thermal stability test results (right).
[Reference Information]
- Paper title / Journal: Approaching the theoretical density limit of ultrahigh-nickel cathodes via cation-disorder-free 10-μm single-crystalline particles, Nature Energy
- DOI: https://doi.org/10.1038/s41560-025-01909-3
[Contact Information]
Prof. Kisuk Kang, Department of Materials Science and Engineering, Seoul National University / +82-2-880-7088 / matlgen1@snu.ac.kr